Newly discovered genetic defect disrupts blood formation and immune system
(Vienna, 21.06.2023) In the quest to find the origin of the puzzling symptoms in four children, researchers from St. Anna Children’s Cancer Research Institute, the CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences (ÖAW), and the Medical University of Vienna have discovered a completely new disease, linking disruptions of blood formation, the immune system, and inflammation. This groundbreaking discovery provides the basis for a better understanding of similar diseases. It is a milestone that the researchers have now published in a prestigious international journal, the New England Journal of Medicine.
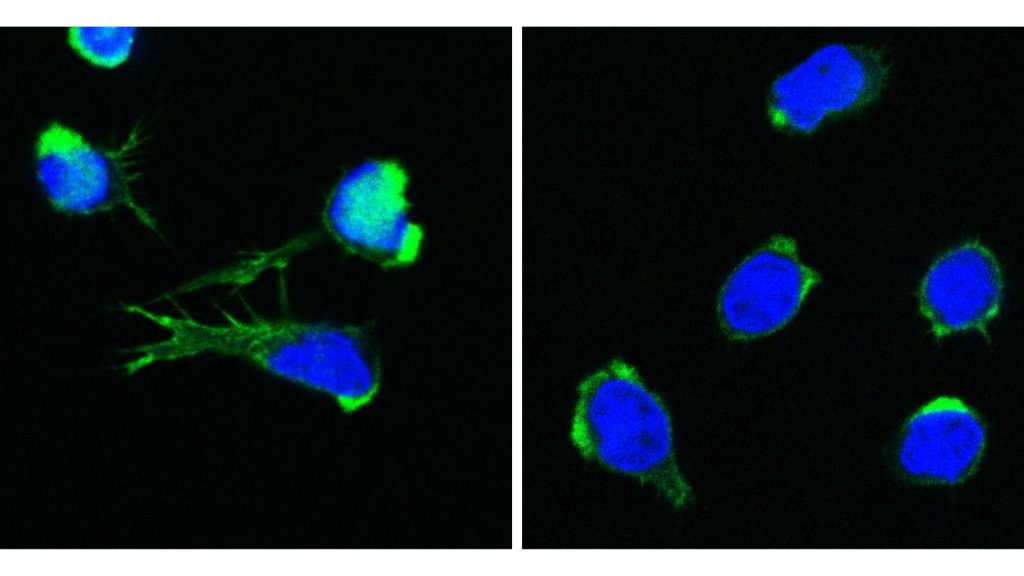
A newly identified defect in the DOCK11 gene leads to abnormalities in both white and red blood cells. “Such rare and previously unknown diseases provide valuable insights into the fundamental principles of blood formation and the immune system. They allow us to better understand the dysregulated processes underlying these diseases,” explains Kaan Boztug, senior author of the study and Scientific Director of St. Anna Children’s Cancer Research Institute (St. Anna CCRI).
©St. Anna CCRI
The gene defect was first identified in a young patient from Spain, where despite repeated tests, no explanation could be found for the severe inflammation affecting various organs such as the kidneys, intestines, and skin. The treating physician then contacted the St. Anna CCRI with blood samples from the patient. And was successful. The internationally leading center for rare diseases of blood formation and the immune system found the cause. “We work closely with international partner institutions to understand such diseases and help the patients,” says Boztug, who is also a Professor in the field of Pediatrics and Inflammation Research at the Medical University of Vienna and an Adjunct Principal Investigator at the CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences.
Three additional patients with variants in the same gene
The patient’s genome sequencing revealed a severe defect in the DOCK11 gene, a gene that had not been previously associated with any human disease and is involved in cell communication. “Initially, we had only one patient and therefore did not know which symptoms were related to the gene defect and which were just additional accompanying manifestations. That was quite a challenge,” says Jana Block, first author of the publication and a PhD student in Boztug’s research group at St. Anna CCRI and CeMM. Through their international collaborations, the researchers managed to find additional patients with similar DOCK11 mutations, which helped provide a clearer understanding of the disease.
DOCK11 controls blood formation
One of the patients suffered from a significantly reduced number of red blood cells and required regular blood transfusions. “Anemia often occurs with immune deficiencies, and often, a person’s own red blood cells are destroyed by autoantibodies that target their own blood cells. In healthy people, however, this does not happen,” says Jana Block. “However, in our investigations, we did not find such autoantibodies.” The cause of the decreased number of red blood cells was clarified by the researchers using a zebrafish model. The DOCK11 defect resulted in impaired blood cell formation, leading to a novel mechanism for anemia, a deficiency of red blood cells.
DOCK11 keeps T cells in check
The role of DOCK11 in humans was, until now, unexplored; previous studies had shown the importance of the protein for the development of B cells in mouse models. The researchers have now shown that to a certain extent B cells also do not develop properly in humans with DOCK11 deficiency, and at the same time, other immune cells, called T lymphocytes, are overactivated. “The protein appears to play a role in keeping the activation of T cells within a certain range,” explains Block. This is important because overactivation can lead to damage to surrounding tissues and organs, especially in the absence of an actual pathogen. A connection between T-cell defects and predisposition to tumor diseases is observed in other immune deficiencies and cannot be ruled out in the case of DOCK11 deficiency. “We will certainly investigate this question further in our research,” says Boztug.
Stem cell transplantation as a possible treatment
Although not all details of DOCK11’s function are yet understood, the researchers suspect that transplantation of blood-forming stem cells could cure the disease. “Of course, these questions need to be investigated in future studies. It is also conceivable that DOCK11 deficiency could be treated through gene therapy,” says Boztug. The fact that there is now hope for the development of therapies has a very special significance for the scientists: “Our work over the past five years is undoubtedly a significant milestone, but we are also aware that several of the patients have died from the disease,” says Block. The researcher hopes that now other centers will become aware of this gene mutation and further advance the exploration of this disease. “This disease is truly severe, so we see it as our mission not only to understand the biological processes but also to develop effective therapeutic strategies based on that,” adds Boztug.
Download press release: