Innovative Cancer Models and ZANDR-Platform
Background
Cancer is a complex disease and is ideally studied in a natural environment as it is greatly influenced by cell interactions, but also by mechanical cues from the surrounding tissue.
The zebrafish is a vertebrate model organism gaining increasing attention in cancer research. Offering fantastic live imaging abilities, zebrafish allow us to follow cancer cells within the intact organism and monitor interactions with other cell types at great detail. Furthermore, zebrafish are well suited to carry out preclinical drug screens in vivo. We make use of these advantages and model pediatric cancer in zebrafish to better understand tumor onset and progression.
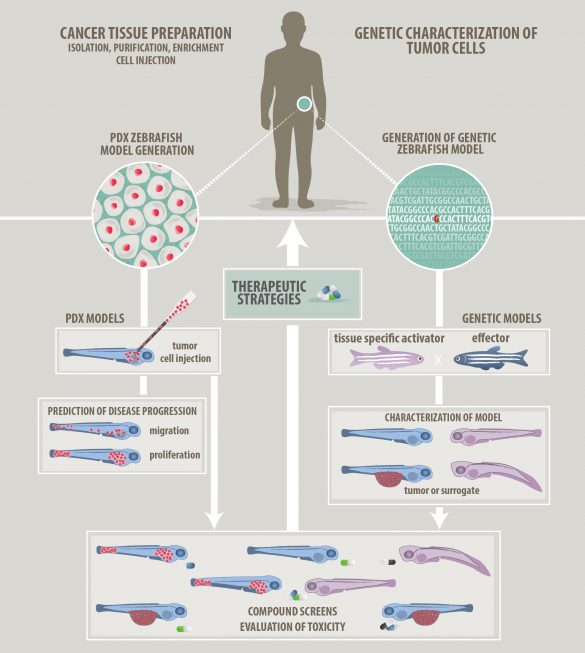
Research approach
To model pediatric cancer, we follow two complementary approaches: a genetic modelling and a xenotransplantation strategy (Figure 1). In our models, we are able to observe tumor cell behavior down to the subcellular level by intravital microscopy and we can capture interactions with the tumor microenvironment. Here, we have a particular interest in interactions with innate immune cells to investigate their pro- and anti-tumor roles.
Zebrafish models of pediatric sarcomas
Ewing sarcoma and osteosarcoma are the most frequent bone cancers found in children and young adolescents with dismal outcome, especially for patients with metastasis or after relapse. We are applying zebrafish in innovative ways to tackle open questions like: What is the cell of origin in Ewing sarcoma? What are main drivers of metastasis in Osteosarcoma?
We have successfully established zebrafish xenograft models for Ewing sarcoma and Osteosarcoma (Figure 2). We our now applying these models in drug screens to identify novel therapeutic strategies.
Figure 1: Strategies to model cancer in zebrafish
The Zebrafish platform Austria for preclinical drug screening (ZANDR) was established at the Children´s Cancer Research Institute (CCRI) by the Distel laboratory in 2019 and is a unique platform to phenotypically screen small compounds on zebrafish models of human diseases.
We leverage the excellent imaging and drug screening possibilities of zebrafish to unravel pediatric cancer etiology and disease-driving mechanisms and to develop novel therapeutic strategies.
Zebrafish and in particular zebrafish larvae are a well suited vertebrate model system to investigate various human diseases and to perform cost-effective screens for small molecules with therapeutic potential (1). Funded by FFG, we have established a zebrafish-optimized and automated screening platform, which is open to the scientific community. Please contact us, if you are interested in collaboration.
(Reference 1: MacRae CA & Peterson RT (2015), Zebrafish as tools for drug discovery, Nat Rev Drug Discov. Oct;14(10):721-31. Doi: 10.1038/nrd4627
 You can find more information here:
You can find more information here:
www.zandr-ccri.at
In vivo drug screening
We have developed a drug screening platform – Zebrafish platform Austria for preclinical drug screening (ZANDR), which is specifically designed to screen small compounds on zebrafish disease models in an automated fashion (see www.zandr-ccri.at). Our recent screen identified compounds and particular compound combinations, which are highly effective against Ewing sarcoma cells in our xenograft setting. We anticipate, that drug screening in zebrafish will provide valuable information to decide, which compounds to advance towards clinical application.
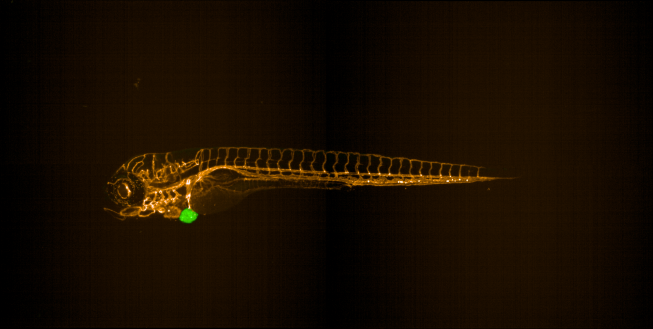
Figure 2: Zebrafish Ewing sarcoma xenograft

Technology development
We are striving to enhance the available tools and methods for zebrafish research with a current focus on
- bringing new imaging modalities to zebrafish
- generating signaling pathway reporter strains in zebrafish
- automating the small compound screening workflow for zebrafish
- adapting non-neural optogenetic and photopharmaceutical tools for zebrafish and applying them in cancer research
Projects and Funding

- Establishing light-mediated clonal cancer models to investigate tumor initiation
CCRI responsible researcher: Adam Varady (supervisor: Martin Distel)
Grant from the Austrian Academy of Sciences (ÖAW), DOC fellowship, ID – 25931
Duration: 01/08/2021 to 01/02/2024

- Tracking Ewing sarcoma origin by developmental and trans-species genomics (ORIGIN)
CCRI responsible Principal Investigator and Coordinator: Heinrich Kovar
Additional CCRI Principal Investigators: Martin Distel, Florian Halbritter
Alex´s Lemonade Stand Foundation (ALSF), Crazy 8 Initiative Award Program
Duration: 01/03/2021 to 28/02/2025



