Background
T cells engineered to express Chimeric Antigen Receptors (CAR T cells) have shown impressive clinical success for patients with B cell malignancies. However, since CAR T cells are self-replicating living drugs it is difficult to regulate their function after administration to a patient, often resulting in severe side effects such as cytokine release syndrome and neurotoxicity. At the same time, currently used CAR T cells could also potentially attack healthy tissue, since their typical target antigens are always present to some extent on a small fraction of healthy cells. This lack of tumor specificity and the insufficient controllability of CAR T cell function are major hurdles for the clinical implementation of the full potential of CAR T cell therapy until today.
CD-Lab Website
For more information see: https://christian-doppler.ccri.at/
Our Research
Tackling self-replicating living drugs
Immunotherapy with CAR T cells has shown impressive clinical success for patients with B cell malignancies. However, since CAR T cells are self-replicating living drugs, it is difficult to regulate their function after administration to a patient, often resulting in severe side effects.
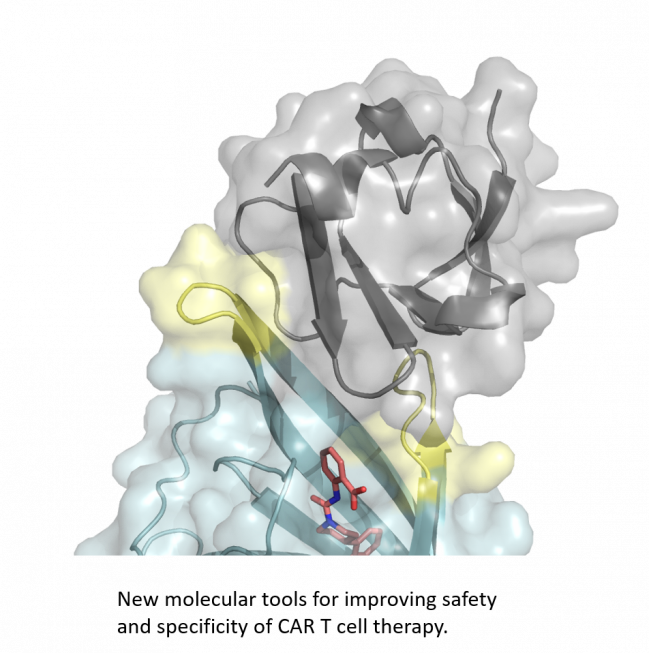
At the same time, currently used CAR T cells could also potentially attack healthy tissue, since their typical target antigens are always present to some extent on a small fraction of healthy cells. This lack of tumor specificity and the insufficient controllability of CAR T cell function are, to date, major hurdles for the clinical implementation of the full potential of CAR T cell therapy.
Increasing controllability of CAR T cells
In November 2019, the Christian Doppler (CD) Laboratory for Next Generation CAR T Cells was launched. In this CDL, we are working together with the University of Natural Resources and Applied Life Sciences and the industrial partner Miltenyi Biotec on solutions to increase the tumor specificity and controllability of CAR T cells – with the ultimate goal of developing new therapeutic options for high-risk childhood cancer.
For more information see: https://christian-doppler.ccri.at/
Selected Articles
Projects and Funding

- CD Laboratory for “Next generation CAR-T cells”
Head of CD Laboratory and Coordinator: Manfred Lehner
Christian Doppler Association, Christian Doppler Lab
Duration: 01/11/2019 to 31/10/2026

- Regulating CAR T cells with a safe and naturally occurring drug
CCRI responsible researcher: Elise Sylvander (supervisor: Manfred Lehner)
Grant from the Austria Academy of Sciences (ÖAW), DOC fellowship, ID – 26323
Duration: 01/07/2022 to 01/07/2024

- Improving tumor specificity of cellular immunotherapies CCRI responsible researcher: Manfred Lehner Grant from the Austrian Science Fund (FWF), Stand-Alone Project, ID – PAT8789924 01/11/2024 – 31/10/2028
Collaborating Partners
Project Partner: Michael Traxlmayr, BOKU-University of Natural Resources and Life Sciences, Vienna, Austria, Head of External Module
Industry Project Partner: Jörg Mittelstaet, Miltenyi Biotec, Germany, Industry Partner






