Background
LCH is a rare histiocytic disorder that may affect any age group, but its most severe clinical course predominantly affects young children. LCH has a wide spectrum of clinical manifestations ranging from single bone lesions, which often regress spontaneously, to severe, sometimes life-threatening multi-system disease, which requires intensive therapy. The etiology of LCH is not known, although it is currently regarded as MAPK-pathway-driven myeloid neoplasm.
Our research
Cellular heterogeneity in LCH
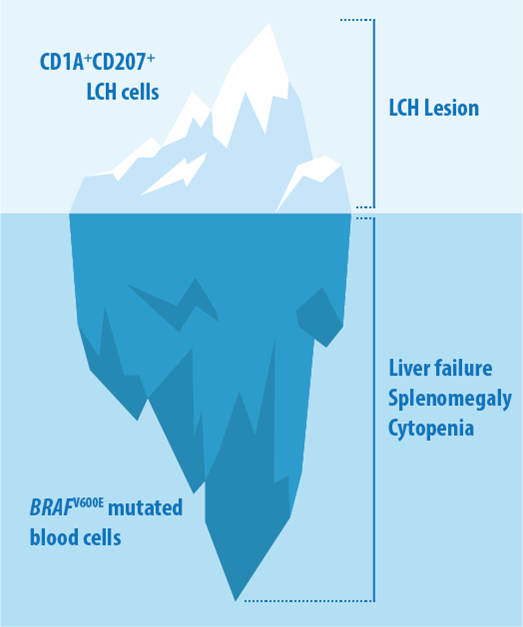
We are particularly interested in the way LCH lesions are composed. We have shown a considerable cellular heterogeneity within LCH lesions and found that LCH cells are plastic and organized in a hierarchical structure. We are dissecting the composition of LCH samples by applying next-generation sequencing techniques, flow analysis and imaging methods. In addition to tumor samples, we are analyzing peripheral blood of patients diagnosed with LCH to characterize circulating cells associated with this disease. By using this approach, we found evidence that BRAF-mutated cells other than the typicial langerin-expressing LCH cells contribute to disease phenotype. BRAF-mutated blood cells other than the typical CD1A+CD207+ cells might contribute to disease in LCH.
Preclinial models for LCH
We aim to devise disease models to ask mechanistic questions on LCH development and to test treatment approaches. We are using peripheral blood monocytes which can be differentiated in the presence of Notch ligands in LCH-like cells. This system can be used to analyze signaling pathways involved in disease induction. In addition, we are employing induced pluripotent stem cells (iPSCs) to characterize how BRAFV600E mutation drives LCH pathogenesis and identify molecular players downstream of treatment with BRAF and ERK inhibitors. In addition, in collaboration with the Distel group, we are testing the effect of BRAFV600E in zebrafish macrophage precursors and in hemtatopoietic stem cells on hematopoiesis and whether we can recapitulate facets of LCH biology in zebrafish. Develop biomarkers for LCH based on peripheral blood BRAFV600E levels In an important translational project, we aim to develop biomarkers that can be used to determine high-risk disease at time of diagnosis and to evaluate treatment response. To do this, we are tracking the level of LCH-associated mutations in the peripheral blood of patients with LCH at time of diagnosis and during treatment. If successful, these biomarkers would fundamentally change diagnosis and enable targeted treatment of children with LCH. To this end, we are collaborating closely with investigators and clinicians worldwide.
Selected Articles
About Caroline Hutter
DDr. Caroline Hutter (MD, PhD), is attending physician in pediatric oncology at the St. Anna Children’s Hospital and Principal Investigator at the St. Anna Children’s Cancer Research Institute, Vienna, Austria. After earning a MD from the Medical University in Vienna, Hutter obtained her PhD at Imperial Cancer Research Fund / Cancer Research UK in London, where she worked in the lab of Fiona Watt to investigate the role of integrins in stem cells. She than joined the lab of Meinrad Busslinger at the Research Institute of Molecular Pathology (IMP) in Vienna and trained in pediatrics at the Medical University and the St. Anna Children’s Hospital. Her special interest lies in precision oncology, hard-to-treat cancers and histiocytoses. Her laboratory research focuses on the pathogenesis and treatment of Langerhans Cell Histiocytosis.







