Breakthrough in Langerhans Cell Histiocytosis Research: Stem Cell Model Paves the Way for New Therapies
(Vienna, 16 December 2024) Scientists at St. Anna Children’s Cancer Research Institute (St. Anna CCRI) have achieved a milestone in the study of the rare and complex disease Langerhans Cell Histiocytosis (LCH). Using an innovative model based on induced pluripotent stem cells (iPSCs), they were able to comprehensively study the mechanisms of the disease for the first time. The groundbreaking results, published in the journal Blood, offer hope for new treatment strategies for those affected.
Langerhans Cell Histiocytosis (LCH) is a rare and complex disorder of the hematopoietic system, characterized by a wide range of symptoms, from self-limiting lesions to tumor-like damage in multiple organs, systemic inflammation, and progressive neurodegeneration. Until now, the lack of suitable models has severely limited research into the disease’s mechanisms.
A pioneering new study, published in the journal Blood, provides critical insights into the mechanisms of LCH and potential treatment strategies. A team led by Caroline Hutter—Group Leader at St. Anna CCRI, Medical Director of St. Anna Children’s Hospital, and Professor of Pediatric Oncology at MedUni Vienna—has successfully developed an in vitro model of LCH. By leveraging an innovative laboratory-developed model based on iPSCs, the need for animal testing has been eliminated.

Innovative Stem Cell Model: A Breakthrough in Research
To develop the model, the researchers introduced the BRAFV600E mutation into human stem cells in the laboratory. This mutation, which is the most common genetic alteration in LCH, triggers changes in cell development, causing the cells to behave similarly to those found in LCH-associated tissue damage.
“Our research highlights how the BRAFV600E mutation drives key characteristics of LCH, including inflammatory responses and neurodegenerative damage,” says Caroline Hutter. “The iPSC model fills a critical gap in LCH research, allowing us to analyze the molecular mechanisms of disease progression in different cell types.” Co-senior author Sebastian Eder, clinical scientist and pediatric oncologist at St. Anna Children’s Hospital, adds, “This model provides an invaluable tool for studying disease mechanisms and testing new treatments.”
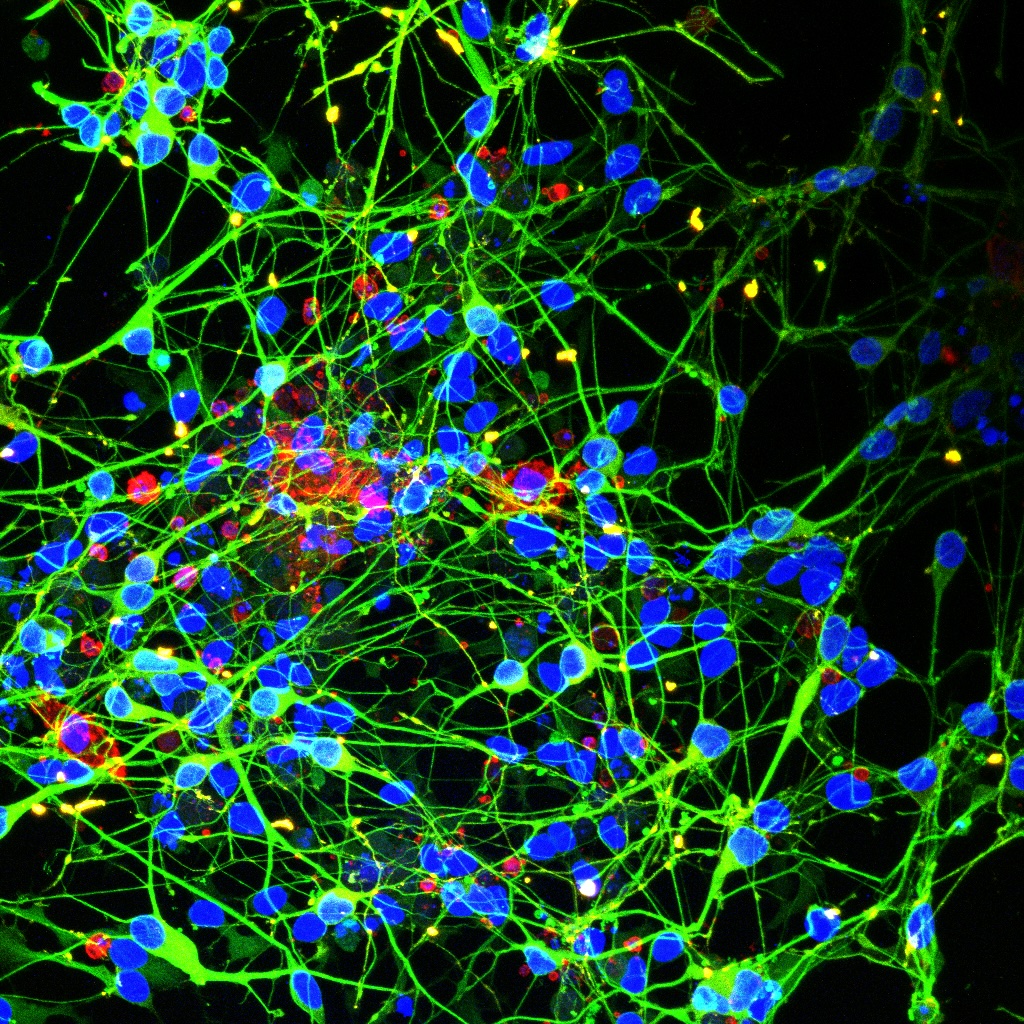
From Precursor Cells to Pathological Tissue Damage
Using their model, the researchers demonstrated that the BRAFV600E mutation causes profound changes during hematopoiesis (blood formation). It alters the way certain genes are read and utilized—a process known as transcriptional regulation. These changes cause specific precursor cells in the blood to develop into cells resembling those found in the diseased tissues of LCH patients.
Reversing Molecular Damage
A particularly significant breakthrough was showing that these disease-related changes are reversible. Using specialized drugs called MAPK pathway inhibitors (MAPKi), the molecular disturbances in the cells were reversed. This finding suggests that these drugs could potentially benefit LCH patients.
Mutant Microglia Drive Neurodegeneration: New Insights into LCH Complications
The team also investigated the interplay between mutant microglia (a type of immune cell in the brain) and neurons, revealing how the BRAFV600E mutation drives neurodegeneration. They found that these mutant microglia cause significant damage to neurons and release substances that serve as markers for neurodegeneration. “Neurodegeneration is currently the most severe complication in the treatment of LCH,” explains Raphaela Schwentner, co-first author of the study. “With this system, we can study interactions between various cell types, such as neurons that are otherwise difficult to investigate, and hopefully develop new therapeutic approaches.”
This study represents a significant advance in understanding LCH and offers new hope for patients with severe and treatment-resistant forms of the disease. Through cutting-edge stem cell technology, the researchers have created a versatile tool for mechanistic studies and drug development. “Our model demonstrates the versatility of iPSCs in translational research,” says Giulio Abagnale, co-first author of the study. “We hope our work will improve the lives of LCH patients and their families.”
Publication
Abagnale G*, Schwentner R*, Ben Soussia-Weiss P, van Midden W, Sturtzel C, Pötschger U, Rados M, Taschner-Mandl S, Simonitsch-Klupp I, Hafemeister C, Halbritter F, Distel M, Eder SK#, Hutter C#. BRAFV600E induces key features of LCH in iPSCs with cell type-specific phenotypes and drug responses. Blood. 2024 Dec 4:blood.2024026066.
doi: 10.1182/blood.2024026066.
(*Co-Erstautoren, #Co-korrespondierende Autoren)