Automated testing of pediatric cancer therapies
(Vienna, 29.6.2023) With a novel High-Throughput-Screening method the efficacy of numerous drugs can be tested simultaneously. Researchers are now able to quickly and efficiently assess which substances are effective against certain tumors. A team led by Martin Distel, PhD, and Sabine Taschner-Mandl, PhD, of St. Anna Children’s Cancer Research Institute has now provided the first guide on how to use this method to test the sensitivity of childhood tumors to different drugs in zebrafish models. The study was published in the Journal npj Precision Oncology.
The recent scientific publication serves as a guide for the treatment of a wide variety of tumors. “In our work, we collaborated with renowned laboratories around the world and gathered information to establish a standardized workflow. A standardization like this did not exist before,” says Martin Distel, co-corresponding author of the study.
A collaboration with Slovenian chemists led to the testing of a new compound in a zebrafish model for sarcomas. “Our tests show that this new compound, an inhibitor of certain heat-shock proteins, actually works slightly better than its predecessors. Based on this finding, we are now fine-tuning the compound further, and a follow-up project is already planned,” reports Sarah Grissenberger, PhD, co-first author of the study and a postdoctoral fellow in Distel’s team.

The foundation for the work: human tumor cells are transplanted into zebrafish larvae in order to test cancer drugs. Tumor development in zebrafish larvae takes only a few days and can be monitored directly, as the fish larvae are transparent. However, in this process precision work is crucial . “Whether the tumor grows also depends on where in the larva we insert the cancer cells,” explains Caterina Sturtzel, PhD, co-first author of the study and research associate in Martin Distel’s Zebrafish Platform Austria for preclinical drug screening (ZANDR). What exactly needs to be taken into account and how to evaluate growth mechanically has now been described in detail by a collaboration of Martin Distel’s and Taschner-Mandl’s teams at St. Anna Children’s Cancer Research Institute.
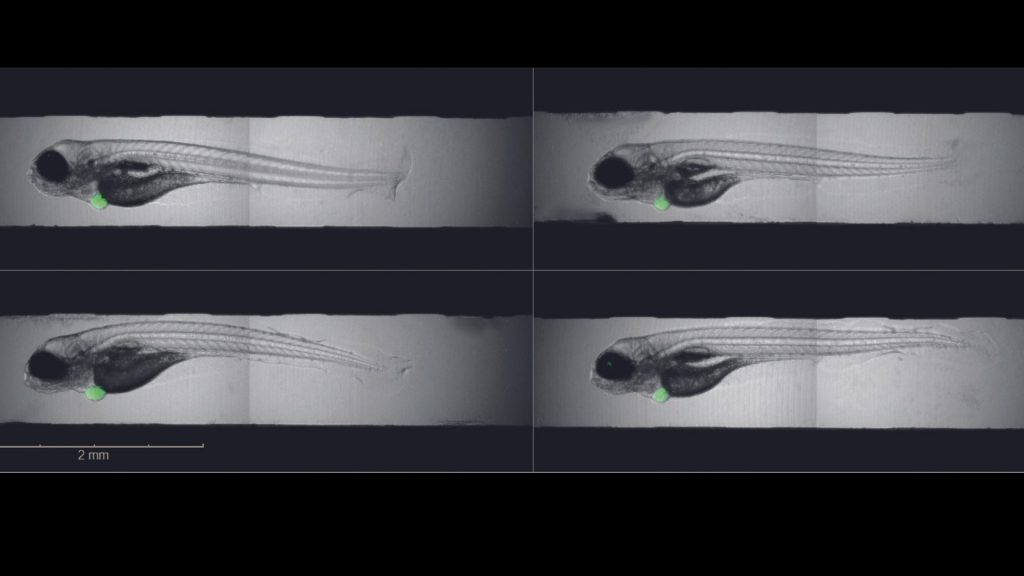
Reliable analysis at the push of a button
In addition to leukemias, certain soft tissue, bone and brain tumors, the team also focused on childhood nerve tumors, so-called neuroblastomas. “Unlike Ewing sarcomas, for example, neuroblastomas don’t always grow equally well. We therefore have to compare each tumor on day 1 and day 3 to assess growth. To do this, we have programmed an analysis workflow that detects the tumor and performs this comparison between day 1 and day 3 in an automated way,” Grissenberger explains. This process is implemented for the first time worldwide by a high content imager called Operetta CLS.
Taschner-Mandl, co-corresponding author and head of the Tumor Biology Group at St. Anna Children’s Cancer Research Institute, comments, “We have seen that different types of neuroblastoma respond differently to the tested drugs ceritinib and temozolomide. This is very important because for many genetic types of neuroblastoma, there are no other animal models in which clinically relevant drug combinations can be tested so quickly.” For the best possible results, the tumor cells must be transplanted exactly where they grow during the disease. Sarah Grissenberger grappled with how best to stain the cells so that the dye would stain only the tumor cells and make them detectable by the high content imager. “Initially, we had a dye that was lost by the cancer cells and picked up by other cells. That can be misleading because we thought: there are metastases everywhere. But with the current dye, that no longer happens and we get reliable results.”
—
Publication
Refined high-content imaging-based phenotypic drug screening in zebrafish xenografts
C. Sturtzel#, S. Grissenberger#, P. Bozatzi, E. Scheuringer, A. Wenninger-Weinzierl, Z. Zajec, J. Dernovšek, S. Pascoal, V. Gehl, A. Kutsch, A. Granig, F. Rifatbegovic, M. Carre, A. Lang, I. Valtingojer, J. Moll, D. Lötsch, F. Erhart, G. Widhalm, D. Surdez, O. Delattre, N. André, J. Stampfl, T. Tomašič, S. Taschner-Mandl* & M. Distel*
#contributed equally
*Corresponding Authors
npj Precision Oncology 2023; May 18; 7:44; DOI: 10.1038/s41698-023-00386-9
Refined high-content imaging-based phenotypic drug screening in zebrafish xenografts | npj Precision Oncology (nature.com)
Funding
This work was supported by the Austrian Research Promotion Agency (FFG), the Alex’s Lemonade Stand Foundation, the Vienna Science and Technology Fund (WWTF), the Slovenian Research Agency, the Federal Ministry of Education, Science and Research, a DOC grant from the Austrian Academy of Sciences, and donations to St. Anna Children’s Cancer Research. This project also received support from the EuropeanH2020-lMI2-JTl-201 5-07 funding. Sanofi sponsored the drug testing on Ewing sarcoma xenografts. The Comprehensive Cancer Center of MedUni Vienna and AKH Vienna co-supported the glioblastoma experiments.
About drug screening in zebrafish larvae
Zebrafish larvae support the search for drugs to treat childhood cancers. Tumor development in fish larvae takes only 24 hours and can be followed in real time in the transparent organism. Researchers at St. Anna Children’s Cancer Research Institute have now developed a working process that allows the semi-automated testing of up to twelve (combination) therapies against tumor cells in just one week. The researchers transplant tumor cells from patients or cell lines into fish larvae. After 24 hours, a tumor develops, which is then treated immediately. After another two days, the effect can already be seen. This means that potential new therapies can be narrowed down considerably at a very early stage, which saves a lot of time. The fact that it is possible at all to test many drugs in such a short time in a living organism is an important step forward. There are only a few laboratories in the world that are able to perform such screenings.
About neuroblastoma
Neuroblastoma is the most common solid tumor outside the brain in children. High-risk neuroblastomas are those tumors that have MYCN amplification or metastatic tumors from eighteen months of age. Unfortunately, the prognosis for this group is still unsatisfactory, with only about half of children with high-risk neuroblastoma surviving the disease long-term. Current standard treatment includes chemotherapy, surgery, autologous stem cell transplantation, and isotretionin in combination with immunotherapy. New approaches are urgently needed.
About Martin Distel
Martin Distel (PhD) graduated in Molecular Biotechnology from the Technical University of Munich, Germany, and Lund University, Sweden. He did his PhD at Helmholtz Centre um Munich under the supervision of Reinhard Köster and developed genetic gene expression tools to study the development of the cerebellum in zebrafish. He also worked with Daniel Razansky at Helmholtz Centre Munich to develop opto-acoustic imaging for zebrafish. As a postdoctoral researcher, he worked in David Traver’s lab at the University of California, San Diego, on zebrafish hematopoiesis.
In 2014, Distel joined St. Anne’s Children’s Cancer Research Institute, where he established a zebrafish laboratory and facility. Since 2017, he has also been the head of the zebrafish platform Austria for preclinical drug screening at St. Anna Children’s Cancer Research Institute.
About Sabine Taschner-Mandl
Sabine Taschner-Mandl (PhD) has headed the Tumor Biology group at St. Anna Children’s Cancer Research Institute since 2018, where she has worked as a scientist since 2008. In addition, the researcher holds a teaching position at the Medical University of Vienna as well as the Vienna University of Technology. Taschner-Mandl completed her biology studies at the University of Vienna with a diploma thesis in vaccine development at Intercell. This was followed by a dissertation and a post-doctoral position at the Institute of Immunology at the Medical University of Vienna. In addition to her work at St. Anna Children’s Cancer Research, Taschner-Mandl was a visiting scientist at Significo and the University of Helsinki as part of the EC-FP7 Marie Curie Program. Taschner-Mandl has received numerous grants for her research, including from the Austrian Research Promotion Agency, the Vienna Science Research and Technology Fund, and the European Commission’s ERA-NET initiative.