“1000 Ideas” to boost childhood cancer research: St. Anna CCRI earns two grants in competitive FWF program
Two projects from St. Anna Children’s Cancer Research Institute (St. Anna CCRI) receive funding from the “1000 Ideas Programme” of the Austrian Science Fund (FWF). The aim of this program is to support daring and original research ideas that lie outside the current scientific understanding. In the second call for funding in this program, Eleni Tomazou, PhD, and Florian Halbritter, PhD, together with Martin Distel, PhD, from St. Anna CCRI were among 22 awardees from a total of 270 applicants.

Recently, the Austrian Research Fund identified an area that has been largely neglected to date, namely high-risk ideas that may be too premature to have good chances of obtaining funding via existing grant programs due to unconventional design or lack of validating data. The FWF’s “1000 Ideas Programme” funds exactly such projects, characterized by a high risk of failure but also comprising great innovative potential. Among the recent funding grants, two projects from St. Anna CCRI were selected, one from Eleni Tomazou and one from Florian Halbritter and Martin Distel. Since childhood cancers are rare diseases, projects such as from our grant winners are an effective and useful complement to classical, conventional research projects, and may open new paths on the way to improving childhood cancer diagnostics and therapy:
Cracking the ribosome-code of cancer drug resistance
Drug resistance is the biggest challenge in cancer therapy. It exists across all types of cancer and all modes of treatment. Understanding the underlying mechanisms by which cancer cells escape cell death is important for the design of effective and sustainable therapeutic interventions. This project, led by Eleni Tomazou, seeks to establish selective protein translation as a novel mechanism of chemotherapeutic drug resistance. “It is based on the hypothesis that heterogeneity in the composition of ribosomes (the “ribosome code”) affects translation in a cell-specific manner and enables some cancer cells to adapt swiftly to chemotherapy by acquiring a metastable phenotype of drug tolerance,” informs Eleni Tomazou. If confirmed, this hypothesis will establish a new resistance mechanism and open up novel therapeutic strategies to prevent therapy resistance by specific targeting of drug tolerance-conferring ribosomes.
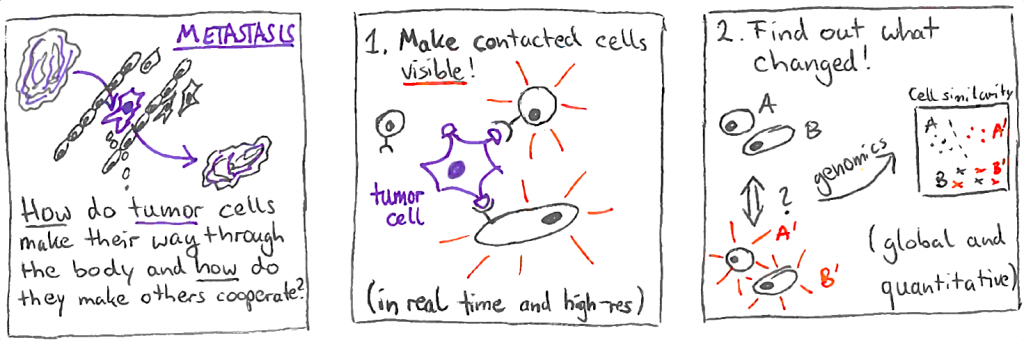
Cell contact tracing to catch metastasis in the act
Cells in humans do not exist in isolation. Interactions between cells critically shape their development and behavior. Yet, the molecular effects of cell contacts remain impossible to track at scale. Florian Halbritter, Martin Distel, and their teams want to elucidate when, where and how tumors manipulate cells to pave their way to forming metastases. To track cell contacts and their effects on the interactors simultaneously, they will develop a system for molecular recording of these contacts by fusing live imaging with deep genomic analysis. The goal of this project is to establish a prototype system for comprehensive cell contact tracing with broad applicability, or a refined blueprint for how such a system could be implemented.
“Our project will provide the means to answer fundamental questions in biomedical research. It is expected to lay the grounds for future, better-informed bioengineering and robust study design. We are happy to make the first step with this ambitious pilot,” says Florian Halbritter.